The first time scientists succeeded in attaching functional molecules with graphene
The first time scientists succeeded in attaching functional molecules with graphene
Germany researchers at the Technical University of Munich (TUM) succeeded to make The first time attaching molecules to the edges of graphene covalently after have succeeded in linking graphene with a chemical group called porphyrins.
These new hybrid structures could also be used in the field of molecular electronics, catalysis or even as sensors.
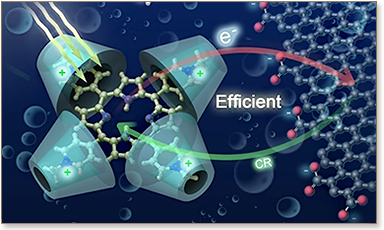
The porphyrin ring structure is aromatic, with a total of 26 electrons in the conjugated system.
What is Porphyrins ?
Porphyrins are a group of heterocyclic macrocycle organic compounds and many porphyrins are naturally occurring; one of the best-known porphyrins is heme, the pigment in red blood cells, a cofactor of the protein hemoglobin, and porphyrins playing a central role in chlorophyll during photosynthesis.
Chlorophyll is a magnesium porphyrin, and heme is an iron porphyrin.
, which are well-known because of their notable functional properties (for instance,
Many previous researchers focus on wet-chemical methods for attaching the molecules to the surface of the material. The TUM team, however, decided to take a different approach: The researchers were able to link porphyrin molecules to graphene in a controlled manner in an ultra-high vacuum using the catalytic properties of a silver surface on which the graphene layer rested. When heated, the porphyrin molecules lose hydrogen atoms at their periphery and can thus form new bonds with the graphene edges.
“This method creates a clean and controllable environment,” explains Professor Auwärter. “We can see exactly how the molecules bond and what types of bonds occur.” Here the researchers use the latest in modern atomic force microscopy to depict the chemical structure of individual molecules, the atomic “skeletons”, so to speak.
Hardly any material is currently receiving as much attention in research as graphene. It is flexible, extremely thin and transparent, while at the same time it has very high tensile strength and conducts electricity, ideal prerequisites for a wide variety of application areas.
The researchers used advanced atomic force microscopy to depict the chemical structure of individual molecules. For the first time the scientists have succeeded in attaching functional molecules to the edges of graphene covalently, i.e. with a stable chemical bond.
The researchers chose the porphyrin molecules as the partner for graphene because of their special properties. The molecules change their properties depending on which metals are at their center and can take on various different tasks, e.g. specifically bonding with gas molecules such as oxygen and carbon dioxide.